Philosophy
 Friedrich August Kekulé von Stradonitz
Friedrich August Kekulé von Stradonitz
September 7th, 1829 to July 13th, 1896
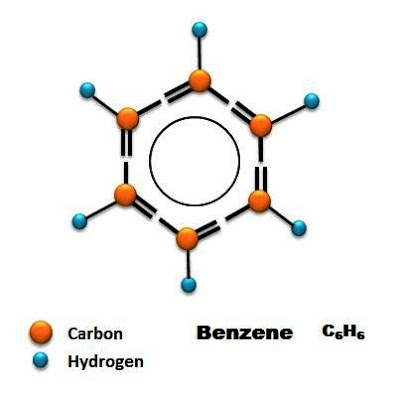
(Friedrich) August Kekulé von Stradonitz was a German chemist who devised the ring structure of carbon atoms in organic molecules. Although at first intending to study as an architect, his career in chemistry began after hearing Justus Liebig's lectures. He determined the tetravalence of carbon, and its ability to link in chains and form polyvalent radicals (1857-58). Further, he envisioned double or even triple bonds between carbon atoms in those chains, and isomers being molecules with the same atoms arranged differently. From a vision of a serpent catching its own tail, Kekulé realized that benzene has a ring structure (1863). Kekulé's ideas became the foundation of structural theory in organic chemistry.
Bill Ashworth wrote in the Linda Hall Library Newsletter...
August Kekulé, a German chemist, died July 13, 1896, at age 66. Kekulé was writing a textbook in organic chemistry in the early 1860s, and was working on a structure for what had recently been called the 'aromatic compounds', which included benzene and its derivatives (not all molecules that are aromatic to the nose are aromatic to the chemist, and some aromatic compounds have no odor at all, but that is another story). In 1865, Kekulé proposed that the benzene molecule was a hexagonal ring of carbon atoms, with alternating single and double bonds, and with a hydrogen atom attached to each vertex of the ring. Benzene derivatives would have different small clusters of atoms, such as a methyl group or hydroxyl radical, attached to the ring instead of hydrogen. Kekulé’s proposed structure was a brilliant suggestion, and it is famous in the history of science became it is one of a half dozen notable discoveries that come attached with a discovery story, like Newton and the apple tree, or Galileo and the tower of Pisa. Kekulé claimed, at a meeting organized in 1890 to celebrate the 25th anniversary of the benzene announcement, that he got the idea of the ring from a day-dream in which he saw a snake biting its tail. Although the story came long after the discovery (which was probably made in 1862), most historians see no reason to doubt it, since it came from Kekulé’s own mouth and was delivered to a gathering of his peers. It is given a little more credence than Newton's apple-tree story, which came over fifty years after the fact, and was recounted by Newton to a young friend, but not written down until 1752. But at least both stories could be true. That is not the case with the tale of Galileo dropping balls from the tower of Pisa, a story that appeared only well after Galileo’s death, and which for a variety of reasons could never have occurred.
Friedrich August Kekulé von Stradonitz [Wikipedia]
- 209 Years Ago Today...dalton's 21 Elements
"Sept. 3, 1803: Dalton Introduces Atomic Symbols" by Randy Alfred September 3rd, 2008 Wired 1803: English chemist-physicist John Dalton starts using symbols to represent the atoms of different elements. Dalton, considered the father...
- Deceased--william N. Lipscomb Jr.
William N. Lipscomb Jr. December 9th, 1919 to April 14th, 2011 "William N. Lipscomb dies at 91; won Nobel Prize in chemistry" The scientist was honored for the first studies to explain the chemistry of boron, in particular the combinations of boron and...
- Nicolas Lémery And "spiky" Atoms
Nicolas Lémery November 17th, 1645 to June 19th, 1715 An early empiricist. His "corpuscular theory" was sophomoric but inventive. French chemist and pharmacist who prepared a comprehensive dictionary of pharmaceuticals in the Pharmacopée universelle...
- Whatever It Takes To Learn...chemistry
Whitney Villanueva using body geometry to represent water's electronic structure. Sometimes the puppets have to be employed to get one's attention and understanding. "High Jinks Boost Chemical Learning" ACS Meeting News: Incorporating games...
- Boron Vs Carbon
Boron: B Atomic Number: 5 Atomic Mass: 10.811 amu Melting Point: 2300.0 °C [2573.15 K, 4172.0 °F] Boiling Point: 2550.0 °C [2823.15 K, 4622.0 °F] Number of Protons/Electrons: 5 Number of Neutrons: 6 Classification: Metalloid Crystal Structure: Rhombohedral...
Philosophy
August Kekulé...father of organic chemistry

 Friedrich August Kekulé von Stradonitz
Friedrich August Kekulé von StradonitzSeptember 7th, 1829 to July 13th, 1896
(Friedrich) August Kekulé von Stradonitz was a German chemist who devised the ring structure of carbon atoms in organic molecules. Although at first intending to study as an architect, his career in chemistry began after hearing Justus Liebig's lectures. He determined the tetravalence of carbon, and its ability to link in chains and form polyvalent radicals (1857-58). Further, he envisioned double or even triple bonds between carbon atoms in those chains, and isomers being molecules with the same atoms arranged differently. From a vision of a serpent catching its own tail, Kekulé realized that benzene has a ring structure (1863). Kekulé's ideas became the foundation of structural theory in organic chemistry.
Bill Ashworth wrote in the Linda Hall Library Newsletter...
August Kekulé, a German chemist, died July 13, 1896, at age 66. Kekulé was writing a textbook in organic chemistry in the early 1860s, and was working on a structure for what had recently been called the 'aromatic compounds', which included benzene and its derivatives (not all molecules that are aromatic to the nose are aromatic to the chemist, and some aromatic compounds have no odor at all, but that is another story). In 1865, Kekulé proposed that the benzene molecule was a hexagonal ring of carbon atoms, with alternating single and double bonds, and with a hydrogen atom attached to each vertex of the ring. Benzene derivatives would have different small clusters of atoms, such as a methyl group or hydroxyl radical, attached to the ring instead of hydrogen. Kekulé’s proposed structure was a brilliant suggestion, and it is famous in the history of science became it is one of a half dozen notable discoveries that come attached with a discovery story, like Newton and the apple tree, or Galileo and the tower of Pisa. Kekulé claimed, at a meeting organized in 1890 to celebrate the 25th anniversary of the benzene announcement, that he got the idea of the ring from a day-dream in which he saw a snake biting its tail. Although the story came long after the discovery (which was probably made in 1862), most historians see no reason to doubt it, since it came from Kekulé’s own mouth and was delivered to a gathering of his peers. It is given a little more credence than Newton's apple-tree story, which came over fifty years after the fact, and was recounted by Newton to a young friend, but not written down until 1752. But at least both stories could be true. That is not the case with the tale of Galileo dropping balls from the tower of Pisa, a story that appeared only well after Galileo’s death, and which for a variety of reasons could never have occurred.
Friedrich August Kekulé von Stradonitz [Wikipedia]
- 209 Years Ago Today...dalton's 21 Elements
"Sept. 3, 1803: Dalton Introduces Atomic Symbols" by Randy Alfred September 3rd, 2008 Wired 1803: English chemist-physicist John Dalton starts using symbols to represent the atoms of different elements. Dalton, considered the father...
- Deceased--william N. Lipscomb Jr.
William N. Lipscomb Jr. December 9th, 1919 to April 14th, 2011 "William N. Lipscomb dies at 91; won Nobel Prize in chemistry" The scientist was honored for the first studies to explain the chemistry of boron, in particular the combinations of boron and...
- Nicolas Lémery And "spiky" Atoms
Nicolas Lémery November 17th, 1645 to June 19th, 1715 An early empiricist. His "corpuscular theory" was sophomoric but inventive. French chemist and pharmacist who prepared a comprehensive dictionary of pharmaceuticals in the Pharmacopée universelle...
- Whatever It Takes To Learn...chemistry
Whitney Villanueva using body geometry to represent water's electronic structure. Sometimes the puppets have to be employed to get one's attention and understanding. "High Jinks Boost Chemical Learning" ACS Meeting News: Incorporating games...
- Boron Vs Carbon
Boron: B Atomic Number: 5 Atomic Mass: 10.811 amu Melting Point: 2300.0 °C [2573.15 K, 4172.0 °F] Boiling Point: 2550.0 °C [2823.15 K, 4622.0 °F] Number of Protons/Electrons: 5 Number of Neutrons: 6 Classification: Metalloid Crystal Structure: Rhombohedral...
